Animal viruses as cloning vectors
The first eukaryotic DNA virus was SV40, for which a complete nucleotide sequence and a detailed understanding of transcription were available. The genome of SV40 contains very little non-essential DNA so it is necessary to insert the foreign gene in place of essential viral genes and to propagate the recombinant genome in the presence of a helper virus. This virus is capable of infecting several mammalian species, following a lytic cycle in some host and a lysogenic cycle in others. The genome is 5.2kb in size and contains two sets of genes, the early genes, expressed early in the infection cycle and coding for proteins involved in viral DNA replication, and the late genes, coding for viral capsid proteins (Figure-18). However, all work using SV40 virions to propagate recombinant DNA molecules is severely constrained by the facts that the viral genome is small, 5.24 kb, and that the packaging limits are strict. Such systems can not, therefore, be used for the analysis of most eukaryotic genes.
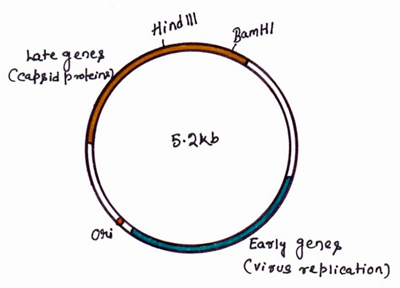
Image source: http://www.discoverbiotech.com/image/image_gallery?uuid=9e863a2c-6cf4-416b-9675-6e5a59388071&groupId=11406&t=1332308806765
Adenoviruses, are a group of viruses which enable larger fragments of DNA to be cloned than it is possible with an SV40 vector. Adenoviruses are more difficult to handle because the genomes are bigger.
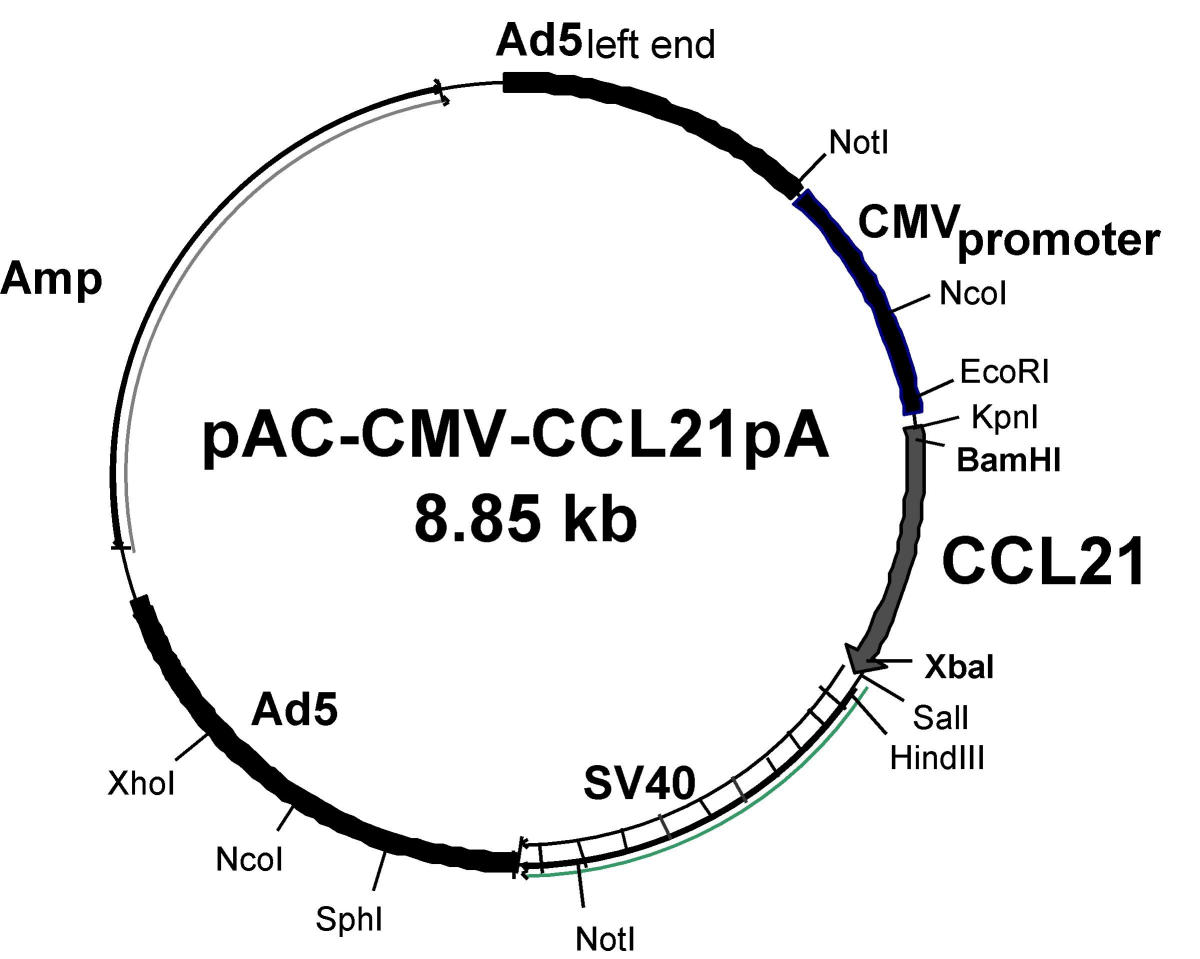
Image source: http://www.molecular-cancer.com/content/2/1/35/figure/F6?highres=y
Papillomaviruses, which also have a relatively high capacity for inserted DNA, have the important advantage of enabling a stable transformed cell line to be obtained. Papillomavirus transformed cells don't contain integrated viral DNA rather they contain between 50 and 300 copies of unintegrated, circular viral DNA although some proportion of these viral genomes exists as concatamers and/or catenates. Bovine papillomavirus(BPV), which causes warts on cattle, has an unusal infection cycle in mouse cells, taking the form of a multi copy plasmid with about 100 molecules present per cell. It doesn't cause the death of the mouse cell, and BPV molecules are passed to daughter cells on cell division. Shuttle vectors consisting of BPV and pBR322 sequences, and capable of replication in both mouse and bacterial cells, are therefore of great value in animal cell biotechnology.
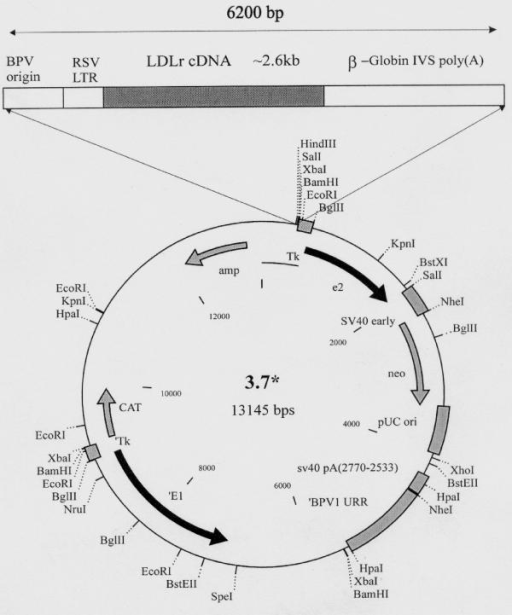
Image source: http://openi.nlm.nih.gov/detailedresult.php?img=111063_1471-2199-3-5-1&req=4
Retroviruses, though have single-stranded RNA genomes but provides perhaps the most promising vector system of all. During the process of reverse transcription, sequences from the termini of viral RNA are duplicated to generate long terminal repeats(LTRs). These long terminal repeats contain both the promoter and the polyadenylation signal for the transcription of viral mRNAs. The specificity of proviral DNA integration is also determined by the long terminal repeats. Although retroviruses can integrate at many sites within the cellular genome, integrative recombination always occurs at particular sites at the ends of the LTRs. The sequences appropriately inserted between the two LTRs will be integrated intact which contrasts sharply with the integration of papovavirus or adenovirus DNA, during which extensive rearrangements of the integrated viral sequences are commonplace. A further great advantage of retroviruses is that they are natural transducing viruses.[26]
Retroviral vectors are most frequently based upon the Moloney murine leukaemia virus (Mo-MLV), which is an amphotrophic virus, capable of infecting both mouse cells, enabling vector development in mouse models, & human cells, enabling human treatment. The viral genes (gag, pol & env) are replaced with the transgene of interest & expressed on plasmids in the packaging cell line. Because the non-essential genes lack the packaging sequence (psi) they are not included in the virion particle. To prevent recombination resulting in replication competent retroviruses, all regions of homology with the vector backbone should be removed & the non-essential genes should be expressed by at least two transcriptional units.[27]
The essential regions include the 5' & 3' LTRs & the packaging sequence lying downstream of the 5' LTR. To aid identification of transformed cells selectable markers, such as neomycin & beta galactosidase, can be included & transgenes expression can be improved with the addition of internal ribosome sites. The available carrying capacity for retroviral vectors is approximately 7.5 kb, which is too small for some genes even if the cDNA is used.[27]
A requirement for retroviral integration & expression of viral genes is that the target cells should be dividing. This limits gene therapy to proliferating cells in vivo or ex vivo, whereby cells are removed from the body, treated to stimulate replication & then transduced with the retroviral vector, before being returned to the patient. When treating cancers in vivo, tumour cells are preferentially targeted However, ex vivo cells can be more efficiently transduced, due to exposure to higher virus titres & growth factors. Furthermore ex vivo treated tumour cells will associate with the tumour mass & can direct tumouricidal effects.[27]
Though transgene expression is usually adequate in vitro & initially in vivo, prolonged expression is difficult to attain. Retroviruses are inactivated by c1 complement protein & an anti-alpha galactosyl epitope antibody, both present in human sera. Transgene expression is also reduced by inflammatory interferons, specifically IFN-alpha & IFN-gamma acting on viral LTRs. As the retroviral genome integrates into the host genome it is most likely that the viral LTR promoters are being inactivated, therefore one approach has been to use promoters for host cell genes, such as tyrosine. Clearly this is an area where continued research is needed.[27]
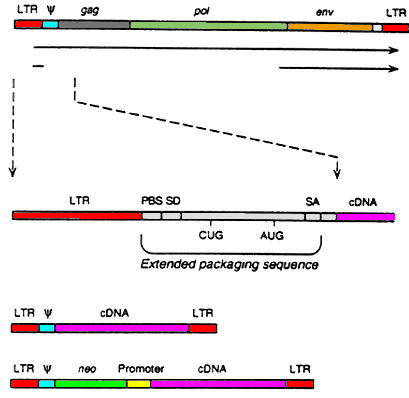
Image source: http://cmbi.bjmu.edu.cn/www-learn/micro-ac-uk/335/peel/peel1.gif
Baculoviruses, enable large amounts of proteins to be obtained from genes cloned in insect cells. One of the major proteins encoded by the virus genome is polyhedrin, which accumulates in very large quantities in the nuclei of infected cells, since the gene has an extremely active promoter. The same promoter can be used to drive the over expression of a foreign gene engineered into the baculovirus genome, and large quantities of protein can be produced in infected insect cells in culture. This method is being used increasingly for large-scale culture of proteins of animal origin, since the insect cells can produce many of the post-translational modifications of animal proteins which a bacterial expression system.
- http://www.discoverbiotech.com/wiki/-/wiki/Main/Cloning+Vectors
- http://www.molecular-cancer.com/content/2/1/35/figure/F6?highres=y
- Tammur J, Sibul H, Ustav E, Ustav M, A
bovine papillomavirus-1 based vector restores the function of the low-density
lipoprotein receptor in the receptor-deficient CHO-ldlA7 cell line,Metspalu
A - BMC Mol. Biol. (2002) .
- http://cmbi.bjmu.edu.cn/www-learn/micro-ac-uk/335/peel/peel2.html